Helen Fisher and J. Anderson Thomson, Jr.
Abstract:
Today millions of people take serotonin-enhancing antidepressants. These medications cause emotional blunting and dysfunction in sexual desire, arousal and orgasm in both men and women. We propose that these antidepressants have other side effects, due to their impact on several related neural mechanisms. Homo sapiens has evolved three distinct brain systems for courtship, reproduction and parenting. The sex drive evolved to motivate men and women to initiate sexual contact with a range of partners; romantic love evolved to motivate them to focus their courtship energy on specific individuals, thereby conserving mating time and energy; partner attachment evolved to motivate our forebears to maintain a stable mateship long enough to rear a child through infancy together. Studies using functional Magnetic Resonance Imaging (fMRI) indicate that romantic love is associated with dopaminergic pathways in the brain’s reward system, pathways that are suppressed by elevated central serotonin. Hence we hypothesize that serotonin-enhancing antidepressants can jeopardize one’s ability to fall in love. Due to their impact on the sex drive, these medications can also jeopardize other brain/body mechanisms that enhance mate assessment, mate choice, pair formation and partner attachment. This paper discusses the biological relationships between the sex drive, romantic love and attachment, as well as other evolved reproductive mechanisms, to illustrate how serotonin-enhancing antidepressants can jeopardize feelings of romance, attachment and fertility.
Download the PDF
LUST, ROMANCE, ATTACHMENT: DO THE SIDE EFFECTS OF SEROTONIN-ENHANCING ANTIDEPRESSANTS JEOPARDIZE ROMANTIC LOVE, MARRIAGE AND FERTILITY?
Helen Fisher and J. Anderson Thomson, Jr.
Helen Fisher, Anthropologist
Dept. Anthropology,
Rutgers University
J. Anderson Thomson, psychiatrist
Counseling and Psychological Services,
Department of Student Health,
University of Virginia
Corresponding Author:
Helen E. Fisher
Office: 4 East 70th Street
New York City, NY 10021
Today millions of people of reproductive age take selective serotonin-reuptake inhibitors (SSRIs) and other serotonin enhancing antidepressants. Approximately 80% of these drugs are prescribed by non-psychiatric physicians, including internists, general practitioners, pediatricians and gynecologists who disseminate them to a wide array of men and women. In the first five months of 2004, American doctors wrote 46 million prescriptions for antidepressants, largely for these drugs (Morais 2004). In the United States alone, antidepressants account for $14 billion a year in wholesale revenues (Morais 2004).
These medications effectively treat a wide range of serious conditions,including major depression, post traumatic stress disorder, generalized anxiety disorders, panic disorders, obsessive compulsive disorder, social phobias, eating disorders, Asperger’s syndrome, irritable bowel syndrome and chronic pain syndromes. But they also produce various side-effects. It is well established that in both men and women these antidepressants can cause emotional blunting, weight gain and several types of sexual dysfunction, interfering with sexual desire, sexual arousal, genital sensation, lubrication, erection, ejaculation and orgasm (Rosen et al 1999; Montejo et al 2001). The number of men and women affected by these forms of sexual dysfunction vary; some studies report that as many as 73% of patients taking serotonin-enhancing antidepressants suffer one or more of these sexual side effects (Montejo et al 2001).
We propose that serotonin-enhancing antidepressants can have far more serious psychological, social and genetic consequences, however, due to their effects on several other neural mechanisms that evolved to enable mate assessment, mate choice, mate pursuit, feelings of romantic love and expressions of attachment to a long term partner.
This paper discusses the neural correlates of the three primary brain systems for courtship, mating, pair formation and reproduction: 1) the sex drive; 2) romantic love; and 3) male/female attachment (companionate love). It explores the neurochemical relationships between these three neural systems to show how serotonin-enhancing antidepressants can potentially jeopardize the ability to fall in love and maintain a stable, long-term partnership. It discusses the potential effects of long-term useof serotonin-enhancing medications on other brain/body mechanisms that evolved to foster courtship and pairbond stability, including penile erection and female orgasm. Last, it illustrates how serotonin-enhancing antidepressants can adversely affect fertility and one’s genetic future.
Three neural systems for mating and reproduction
Neuroscientists currently believe that the basic human emotions and motivations arise from distinct systems of neural activity; that these brain systems derive from mammalian precursors; and that these brain mechanisms evolved to enable survival and reproduction (Davidson 1994; Panksepp 1998). Among these primary neural systems are three discrete, interrelated motivation/emotion systems for mating, reproduction and parenting: the sex drive, romantic love and male/female attachment. Each of these motivation/emotion systems is associated with a different behavioral repertoire; each is associated with a different and dynamic constellation of neural correlates; and each evolved to direct a different aspect of reproduction (Fisher 1998).
The sex drive is characterized by the craving for sexual gratification. It is associated primarily with the estrogens and androgens in non-primate mammalian species. In humans and other higher primates the estrogens have little direct influence on sexual desire (Meston and Frolich 2000); instead the androgens, particularly testosterone, is crucial to sexual desire in both sexes (Edwards and Booth 1994; Sherman 1994; Van Goozen et al 1997). The sex drive evolved principally to motivate individuals to seek sexual union with a range of reproductive partners.
Romantic love (also known as obsessive love, passionate love or being in love) is characterized by intense energy, focused courtship attention, ecstasy, mood swings, sexual possessiveness, emotional dependency, obsessive thinking about the beloved, craving for emotional union with the beloved and intense motivation to win this preferred mating partner (Fisher 1998; Gonzaga et al. 2001; Harris 1995; Hatfield et al. 1988; Hatfield and Sprecher 1986; Shaver et al. 1987; Tennov 1979). Evidence suggests that romantic love is primarily associated with elevated activity in dopaminergic pathways of the reward system of the brain (Aron et al,2005; Bartels and Zeki 2000; Bartels and Zeki 2004); and data suggest that other mammals share central biological and behavioral aspects of this brain system (Gingrich et al., 2000; Wang et al., 1999; Liu and Wang 2003; Fabre-Nys et al 1997). The neural system associated with romantic love evolved to motivate individuals to prefer a specific mating partner, thereby conserving courtship time and energy.
Partner attachment in humans is associated with feelings of calm, security, social comfort and emotional union with a long-term mating partner, as well as with some of the traits of mammalian attachment, including mutual territory defense and/or nest/home building, mutual feeding and grooming, maintenance of close proximity, separation anxiety, shared parental chores and affiliative gestures (Carter et al., 1997, Young, Wang, & Insel, 1998; Lim, Murphy, & Young, 2004; Lim and Young 2004). Animal studies suggest that this brain system is associated primarily with the neuropeptides, oxytocin and vasopressin (Carter 1992; Winslow et al 1993; Lim, Murphy & Young 2004; Lim and Young 2004). Adult male/female partner attachment evolved primarily to motivate individuals to sustain an affiliative connection with a reproductive partner at least long enough to complete species-specific parental duties (Fisher 1992).
We propose that when patients use serotonin-enhancing antidepressants, they can potentially jeopardize not only their sex drive but also these related neural mechanisms for romantic love and partner attachment.
The Sex Drive
The androgens, particularly testosterone, is central to sexual desire in both men and women (Edwards and Booth 1994; Sherman 1994; Van Goozen et al 1997). Individuals who have higher circulating levels of testosterone tend to engage in more sexual activity (Edwards and Booth 1994; Sherwin 1994). Male athletes who use testosterone and other anabolic steroids to elevate strength and stamina have more sexual thoughts, more morning erections, more sexual encounters and more orgasms. Middle-aged women who inject or apply testosterone cream to the skin boost sexual desire. The male libido peaks in the early twenties, when the activity of testosterone is highest. Many women feel more sexual desire around ovulation, when testosterone increases (Van Goozen et al 1997). Both sexes also have fewer sexual fantasies, masturbate less regularly and engage in less intercourse as they age, as testosterone declines (Edwards and Booth 1994). People vary in their degree and frequency of sexual desire, in part because levels of testosterone are inherited (Meikle et al 1988). Moreover, the balance between testosterone, estrogen and other bodily systems, as well as social circumstances, childhood experiences and a host of other factors play a role in when, where and how often one feels lust (Nyborg 1994). Nevertheless, testosterone is central to the sex drive.
The sex drive is also associated with a specific range of neural correlates. Using functional Magnetic Resonance Imaging (fMRI), Arnow and colleagues report that when young male heterosexual subjects look at erotic video material while wearing a custom-built pneumatic pressure cuff around the penis, they show strong activations in the right subinsular region, including the claustrum, the left caudate and putamen, the right middle occipital/middle temporal gyri, the bilateral cingulate gyrus and right sensorimotor and pre-motor regions and the right hypothalamus (Arnow et al 2002). Beauregard and colleagues measured brain activation (using fMRI) in men as they viewed erotic film excerpts (Beauregard et al 2001). Activations occurred in limbic and paralimbic structures, including the right amygdala, right anterior temporal pole and hypothalamus.
Using fMRI, Karama and colleagues also recorded brain activity while men andwomen viewed erotic film excerpts (Karama et al 2002). Activity increased in the anterior cingulate, medial prefrontal cortex, orbitofrontal cortex, insula and occipito-temporal cortices, as well as in the amygdala and the ventral striatum. Men showed activation in the thalamus and significantly greater activation than women in the hypothalamus, specifically in a sexually dimorphic area associated with sexual arousal and behavior. In another experiment, researchers measured brain activity among eight men as these subjects experienced orgasm. Blood flow decreased in all regions of the cortex except one region of the prefrontal cortex where it increased (Tiihonen et al 1994). Animal studies also indicate that several brain structures are associated with the sex drive and sexual expression, including the medial amygdala, medial preoptic area, paraventricular nucleus and periaqueductal gray (Heaton 2000), and the septum and ventromedial hypothalamus (Dixson, 1998).
These data indicate that the constellation of neural correlates associated with the sex drive are dynamic yet specific. Moreover, data on the neural correlates associated with romantic love (see below) indicate that the sex drive and romantic love are overlapping yet distinct neural systems.
The Neural Correlates of Romantic Love
Intense courtship attraction, commonly known as romantic love, isrecorded in all human societies for which data are available (Jankowiak and Fischer 1992), and despite the varied ways that this phenomenon is expressed cross-culturally, this multi-part experience is associated with a specific constellation of motivations and emotions (Fisher 1998; Hatfield and Sprecher 1986; Hatfield et al. 1988; Gonzaga et al. 2001; Shaver et al. 1987; Tennov 1979; Harris 1995).
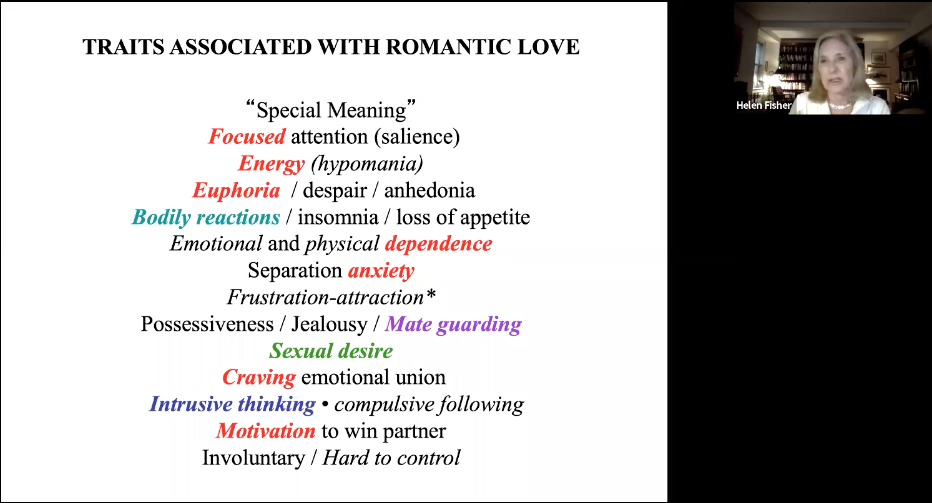
Romantic love generally begins as a person starts to regard another as special, unique. The lover focuses his/her attention on the beloved, doting on the beloved’s worthy traits and overlooking or minimizing their flaws. The lover expresses increased energy, ecstasy when the love affair is going well, and mood swings into despair during times of adversity. Barriers heighten romantic passion, what has been referred to as “frustration attraction” (Fisher 2004). The lover suffers “separation anxiety” when apart from the beloved, and often a host of sympathetic nervous system reactions when with the beloved, including sweating and a pounding heart. Lovers are emotionally dependent; they tend to change their priorities and daily habits to remain in contact with and/or impress the beloved. They exhibit empathy for the beloved; many are willing to sacrifice, even die for this special other. The lover expresses sexual desire for the beloved, as well as intense sexual possessiveness. Yet their craving for emotional union supersedes their craving for sexual union with him or her. Most characteristic, the lover thinks obsessively about the beloved. Rejected lovers generally protest and try to win the beloved back, as well as express “abandonment rage” and despair. Romantic passion is also involuntary, difficult to control and generally impermanent.
To investigate the neural correlates of romantic love, Fisher and colleagues Lucy Brown, Arthur Aron and others used functional Magnetic Resonance Imaging (fMRI) to study the neural activity of ten women and seven men who reported being “madly in love”(Aron et al 2005). Participants’ age range was 18-26 years (M = 20.6; median = 21); subjects reported being in love an average of 7.4 months (median = 7; range 1-17 months).
A preliminary investigation had identified a photograph of the beloved as an effective stimulus for eliciting feelings of intense romantic love (Mashek et al. 2000). So the protocol employed photographs and consisted of four tasks presented in an alternating block design: For 30 seconds each participant viewed a photo of his/her beloved (Positive stimulus); for the following 40 seconds each performed a Countback distraction task; for the following 30 seconds each viewed a photograph of an emotionally neutral acquaintance (Neutral stimulus); for the following 20 seconds each performed a similar Countback task. The Countback task involved viewing a large number, such as 8,421, and mentally counting backwards (beginning with this number) in increments of seven. The Countback task was included to decrease the carry-over effect after the participant viewed the Positive stimulus because it is difficult to quell intense feelings of romantic love. This four-part sequence (or a counterbalanced version beginning with the neutral stimulus) was repeated six times; the total stimulus protocol was 12 minutes.
Group activation specific to the beloved occurred in the right ventral tegmental area (VTA), localized in the region of A10 dopamine cells, and the right medial and postero-dorsal body of the caudate nucleus (Aron et al, 2005). The VTA is rich in cells that produce and distribute dopamine to many brain regions, including the caudate nucleus. The VTA is also a central part of the brain’s “reward system” (Wise 1989; Schultz et al 1997; Schultz 2000; Fiorillo et al 2003; Martin-Soelch et al 2001; Breiter et al 2001), the neural network associated with sensations of pleasure, general arousal, focused attention and motivation to pursue and acquire rewards (Schultz 2000; Delgado et al 2000; Elliot et al 2003; Gold 2003). The caudate nucleus is also associated with reward, motivation and goal-oriented behaviors. It plays a role in reward detection and expectation, the representation of goals, and the integration of sensory inputs to prepare for the appropriate actions to win rewards (Lauwereyns et al. 2002; Martin-Soelch et al. 2001; O’Doherty et al. 2004; Schultz 2000). Some 80% of receptor sites for dopamine reside in the caudate nucleus.
Using functional magnetic resonance imaging (fMRI), Bartels and Zeki (2000) also investigated brain activity in seventeen men and women who reported being “truly, deeply, and madly in love”(2000:3829). Eleven were women; all looked at a photograph of his/her beloved, as well as photographs of three friends of similar age, sex and length of friendship. Individuals reported being in love an average of 28.8 months, however; considerably longer than the love relationships studied by Fisher, Aron, Brown and colleagues, who were in love an average of 7.4 months (Aron et al 2005). Those in the Bartels and Zeki study (2000) were also less intensely in love (Aron et al, 2005). In spite of these differences, Bartels and Zeki (2000; 2004) found that romantic love also activated regions of the caudate nucleus and the ventral tegmental area, as well as several different brain areas.
These combined data support the hypothesis that dopaminergic pathways in the reward system of the brain play a central role in the focused attention and motivation associated with romantic love (Fisher 1998).
Elevated activity of central dopamine is also associated with ecstasy, intense energy, hyperactivity, sleeplessness, mood swings, emotional dependence and craving (Wise, 1988; Wise, 1996; Colle and Wise 1988; Post, Weiss, & Pert, 1988; Kiyatkin 1995; Salamone 1996; Abbott 2002; Schultz et al 1997; Robbins and Everitt 1996), more central traits of romantic love. The addictive behaviors associated with romantic love are most likely related to dopamine activity as well (Fisher 2004), because acute cocaine injection has been shown to activate the VTA in fMRI studies of humans (Breiter et al. 1997); animal studies of cocaine addiction also implicate mesolimbic dopamine pathways (David et al. 2004; Kalivas and Duffy 1998; McBride et al. 1999; Wise and Hoffman 1992),
Norepinephrine may also be associated with human romantic love (Fisher 1998), although this has not yet been recorded by neuroimaging. Increased activity of norepinephrine generally produces alertness, energy, sleeplessness, loss of appetite (Coull et al 1998; Robbins et al 1998) and increased attention (Marracco and Davidson 1996; Posner and Peterson 1990), some of the basic characteristics of romantic love (Tennov 1979; Hatfield and Sprecher 1986; Fisher 2004). Elevated activity of cenral norepinephrine also increases memory for new stimuli (Griffin and Taylor 1995) so this neurotransmitter may also contribute to the lover’s ability to remember the smallest details of the beloved’s actions and cherished moments spent together. As norepinephrine is also associated with sympathetic nervous system responses, including increased heart rate and blood pressure, and these responses often occur in early stage, intense romantic love, norepinephrine may contribute to these aspects of romantic love as well.
Low activity of central serotonin also may be involved in feelings of intense romantic love (Marazziti 1999; Fisher 1998). This is hypothesized because a striking sympton of romantic love is incessant, obsessive thinking about the beloved (Tennov 1979; Hatfield and Sprecher 1986; Fisher 1998; Fisher 2004), and low activity of central serotonin is associated with obsessive-compulsive disorder (OCD) (Insel et al 1985; Insel et al 1990). In fact, most forms of OCD are treated with antidepressants that elevate the activity of central serotonin (Flament et al 1985; Hollander et al 1988; Thoren et al 1980).
A recent study supports the hypothesis that romantic love is associated with low levels of central serotonin. In this experiment, 20 men and women who had fallen in love in the previous six months, 20 suffering from unmedicated obsessive-compulsive disorder (OCD), and 20 normal (control) individuals who were not in love were all tested for plasma levels of serotonin (Marazziti et al 1999). Both the in-love participants and those suffering from OCD showed significantly lower concentrations of the platelet serotonin transporter (Marazziti et al 1999). Although bodily activities of serotonin do not necessarily correlate with serotonin activities in the brain (Kendrick et al 1986), decreased activity of central serotonin may contribute to the lover’s obsessive thinking. As impulsivity is also associated with low activity of central serotonin (Tiihonen et al 1997), decreased activity of this neurotransmitter may also produce the impulsivity associated with romantic love.
These above data suggest that the constellation of neural correlates associated with romantic love are largely distinct from those of the sex drive. Moreover, both neural systems are fundamental human drives (Fisher 2004).
The Drive to Love
Psychologists distinguish between emotions, affective states of feeling, and motivations, brain systems oriented around the planning and pursuit of a specific want or need, and Arthur Aron has proposed that romantic love is not primarily an emotion, but a motivation system designed to enable suitors to build and maintain an intimate relationship with a preferred mating partner (Aron and Aron 1991; Aron et al 1995). Because the above mentioned experi-ments indicate that romantic love is associated with activity in the VTA and caudate nucleus, Aron’s hypothesis is supported: motivation and goal-oriented behaviors are central to the experience of intense, early stage romantic love. In fact, these data suggest that romantic love is a primary motivation system: a fundamental human mating drive (Fisher 2004).
Pfaff defines a drive as a neural state that energizes and directs behavior to acquire a particular biological need to survive or reproduce (Pfaff 1999:7;40). Like drives, romantic love is tenacious; emotions come and go. Like drives, romantic love is focused on a specific reward, in this case the beloved; emotions, such as fear, are associated with a wider range of objects and ideas. Like drives, romantic love is not associated with any particular facial expression; all of the primary emotions have stereotypic facial poses. Like drives, romantic love is difficult to control; it is harder to curb thirst, for example, than to control anger. Last, like all of the basic drives (Pfaff 1999), romantic love is associated with elevated activity in the dopaminergic reward system in the brain.
Drives lie along a continuum (Fisher 2004). Some, like thirst and the need for warmth, cannot be extinguished until satisfied. The sex drive, hunger, the craving for salt and the maternal instinct can often be redirected, even quelled. Falling in love is evidently stronger than the sex drive because when one’s sexual advances are rejected, people do not kill themselves or someone else; rejected lovers sometimes commit suicide or homicide (Meloy and Fisher in press).
Mammalian courtship attraction
Romantic love and the sex drive are not only distinct neural systems, but evidence suggests that they may have been distinct since the proliferation of mammalian species some 70 million years BP. All mammals have mate preferences; none will copulate with anyconspecific (Fisher et al 2002). The drive to pursue a preferred mating partner is so common that the ethological literature regularly uses several terms to describe it, including “mate choice,” “female choice,” “individual preference,” “favoritism,” “sexual choice” and “selective proceptivity”(Andersson 1994).
This mate preference in mammals, referred to as “courtship attraction,” is associated with many of the same characteristics as human romantic love, including heightened energy, focused attention, obsessive following, sleeplessness, lose of appetite, possessive “mate guarding,” affiliative gestures, goal-oriented courtship behaviors and intense motivation to win a specific mating partner (Fisher 2004). Moreover, animal studies indicate that elevated activities of dopaminergic reward pathways play a primary role in mammalian mate preference, data that correlates with the above evidence for the role of dopaminergic pathways in human romantic love.
For example, when a female laboratory–maintained prairie vole (Microtus ochrogaster) is mated with a male, she forms a distinct preference for him associated with a 50% increase of dopamine in the nucleus accumbens (Gingrich et al 2000). When a dopamine antagonist is injected into the accumbens, the female no longer prefers this partner; and when a female is injected with a dopamine agonist, she begins to prefer the conspecific who is present at the time of infusion, even if she has not mated with this male (Gingrich et al., 2000; Wang et al., 1999; Liu and Wang 2003). An increase in central dopamine is associated with courtship attraction in female sheep (Fabre-Nys, 1998). In male rats increased striatal dopamine release has also been shown in response to the presence of a receptive female rat (Montague et al. 2004; Robinson et al. 2002).
In most species, this excitatory state is brief (Fisher 2004); among humans, romantic love can last 12 months or more (Marazziti 1999). Nevertheless, mammalian courtship attraction and human romantic love have much in common, including behavior patterns and neural mechanisms. So it is parsimonious to hypothesize that the neural correlates of courtship attraction developed into those for human romantic love some time during hominid evolution, perhaps along with the development of the hominid brain some two million years BP (Fisher 2004). Moreover, it is likely that this neural mechanism serves the same purpose in all mammalian species: to enable individuals to discriminate between the courtship displays of an array of suitors, prefer those that advertise superior genes, better resources and/or more parental investment, and motivate males and females to focus their courtship attention on these preferred individuals, thereby conserving mating time and energy (Fisher et al 2002; Fisher 2004).
Despite the biological distinctions between romantic love and the sex drive, and what is likely to be their long evolutionary history, the brain systems for the sex drive and romantic love interact in many ways, suggesting that serotonin-enhancing antidepressants can potentially suppress feelings of romatic love.
Interactions between the Sex drive and romantic love
Men and women in Western societies do not generally confuse the ecstasy, focused attention and obsessive thinking associated with romantic love with the mere appetite for sexual release (Tennov 1979; Hatfield and Rapson 1996). Men and women in an array of traditional societies also make this distinction (Jankowiak 1995). On the Polynesian island of Mangaia “real love” is called inangaro kino, a state of romantic passion distinct from one’s sexual desires (Harris 1995). The Taita of Kenya call lust ashiki, while they refer to love as pendo(Bell 1995). In Caruaru, Northeast Brazil, locals say, “Amor is when you feel a desire to always be with her, you breathe her, eat her, drink her, you are always thinking of her, you don’t manage to live without her”(Rebheun 1995:253). Paixao, on the other hand, is “horniness” and tesao is “a very strong sexual attraction for a person” (Rebheun 1995:254).
Despite people’s ability to distinguish between feelings of passionate romantic love and feelings of sexual desire, those who fall in love regularly begin to find their beloved enormously sexually attractive; sexual desire is a central trait of human romantic love. This positive association between romantic love and the sex drive may be due, in part, to the biological link between these two brain systems. Dopamine can stimulate a cascade of reactions, including the release of testosterone and estrogen (Hull et al 1995; Wenkstern et al 1993; Wersinger SR and EF Rissman 2000; Szezypka, Zhou and Palmiter 1998; Hull et al 1997; Kawashima and Takagi 1994); and increasing activity of testosterone and estrogen can promote dopaminerelease(Hull et al 1999; Becker 2001; Creutz, L.M.and M.F. Kritzer 2002; Appararundaram et al 2002; Auger et al 2001; Pfaff 2005).
Animal studies confirm this positive correlation between the sex drive and the dopaminergic arousal system. When a male laboratory rat is placed in an adjacent cage where he can see or smell an estrous female, his levels of central dopamine increase and elevate sexual arousal and pursuit of the female (Hull et al 1995; Hull, et al., 2002; Hull et al 1997; Wenkstern et al 1993; West et al 1992). When the barrier is removed and the male is allowed to copulate, levels of dopamine continue to rise (Hull et al 1995). When dopamine is injected into specific regions of
the brain in male rats, the infusion stimulates copulatorybehavior (Ferrari and Giuliana 1995); and blocking the activities of central dopamine in rats diminishes several proceptive sexual behaviors, including hopping and darting (Herbert, 1996). Pfaff reports that in male rats, dopamine increases male sexual behavior through at least three functional roles. It increases sexual arousal and courtship behavior; it potentiates the motor acts of mounting; and it faciliates genital responses to stimulation (Pfaff 2005).
This positive correlation between central dopamine, the sex steroids and sexual arousal and performance is not only common in animals (Liu et al 1998; Herbert 1996; Pfaff 2005); it also occurs in humans (Clayton et al, 2000; Walker et al 1993; Heaton 2000). When individuals who suffer from hypoactive sexual desire disorder are treated with dopamine enhancing medications, their libido improves (Segraves et al 2001). When patients suffering from depression take drugs that elevate the activity of dopamine, their sex drive often improves as well (Walker et al 1993; Coleman et al 1999; Ascher et al 1995). In fact, some patients who currently take serotonin enhancing antidepressants supplement their therapy with medications that elevate the activity of dopamine (and norepinephrine) solely to maintain or elevate sexual arousal (Walker et al 1993; Coleman et al 1999; Ascher et al 1995; Rosen et al 1999).
Norepineprhine is also positively linked with sexual motivation and sexual arousal (Fraley 2002; Van Bockstaele, 1989; Clayton et al 2002; Pfaff 2005; Etgen and Morales 2002). When a female prairie vole is exposed to a drop of male urine on the upper lip, norepinephrine is released in parts of the olfactory bulb, contributing to the release of estrogen and concomitant proceptive behavior (Dluzen et al 1981); and estradiol and progesterone produce the release of norepinephrine in the hypothalamus to produce lordosis in rats (Etgen et al 1999). Last, when ovariectomized, sexually receptive female rats receive injections of estrogen and are then permitted to mate, copulation produces the release of norepinephrine in the lateral ventromedial hypothalamus (Etgen and Morales 2002).
This positive relationship between norepinephrine and the sex drive may be due, in part, to its interaction with the androgens. Norepinephrine, like dopamine, stimulates the production of testosterone (Mayerhofer et al 1992; Fernandez et al 1975; Cardinali et al 1975); and increasing levels of testosterone can elevate the activity of norepinephrine (Jones et al 1998) and dopamine(Hull et al 1999; Becker 2001; Pfaff 2005). Drug users attest to this positive chemical connection between norepinephrine and the sex drive. In the right oral dose, amphetamines (norepinephrine agonists) enhance sexual desire (Buffum et al 1988).
The above data indicate that romantic love is associated with elevated activity of dopamine (and most likely also norepi-nephrine) in general arousal systems in the brain. Moreover, these catecholamines are positively correlated with sexual motivation and sexual arousal. Most important to this paper, elevated activity of serotonin can directly suppress all pathways for dopamine (Meston and Frohlic 2000; Stahl 2000) and norepinephrine(Done and Sharp 1992), as well as suppress testosterone activity (Netter et al 1998; Sundblad and Eriksson 1997; Gonzalez et al 1994). Hence, serotonin enhancing antidepressants that negatively affect the sex drive and sexual arousal are also likely to adversely affect feelings of romantic love.
Case study: In one case, a 20-year-old, single, white, female undergraduate patient with an eating disorder, recurrent depressions and attention deficit disorder (ADD) was administered an SSRI at relatively high doses for her eating disorder. When asked about side effects, she said she had none. When asked specifically about sexual side effects, she wasn’t certain and asked that they be explained. Once they were explained, she acknowledged that she did have sexual side effects but that she had attributed them to problems in her relationship. “I have not been as much in love with my boyfriend,” she reported. “I am not as interested in intimate time with him. I find myself wanting more space.” At the time she reported this, the dose of the SSRI had just been increased.
Emotional blunting and romantic love
Serotonin-enhancing medications can also jeopardize feelings of romantic love indirectly, by affecting the emotions. A striking characteristic of romantic love is obsessive thinking about a beloved. As discussed above, this intrusive thinking is most likely associated with low activity of central serotonin. Hence individuals taking serotonin-enhancing antidepressants are likely to suppress the obsessive thinking characteristic of romantic love. Elation is another primary feature of romantic love and individuals who take serotonoin-enhancing antidepressants are likely to suppress this ecstasy as well.
Serotonin-enhancing medications are well known to blunt the emotions, and an unsolicited letter to The New York Times in response to our ideas (Fisher and Thomson 2004; O’Connor 2004) illustrates the impact that an SSRI had on Dr. Jerry Frankel, of Plano, Texas. “After two bouts of depression in 10 years, my therapist recommended I stay on serotonin-enhancing antidepres-sants indefinitely. As appreciative as I was to have regained my health, I found that my usual enthusiasm for life was replaced with blandness. My romantic feelings for my wife declined drastically. With the approval of my therapist, I gradually discontinued my medication. My enthusiasm returned and our romance is now as strong as ever. I am prepared to deal with another bout of depression if need be, but in my case the long-term side effects of antidepressants render them off limits” (Frankel 2004).
The drive to attach
Love changes over time; the ecstasy, energy, focused attention, obsessive thinking, yearning, and intense motivation to win the beloved gradually diminish, often transforming into feelings of comfort, calm and emotional union with one’s partner. This male/female partner attachment system is characterized in birds and mammals by mutual territory defense and/or nest building, mutual feeding and grooming, the maintenance of close proximity, separation anxiety, shared parental chores and other affiliative behaviors. In humans, partner attachment is also characterized by feelings of calm, security, social comfort and emotional union with a partner. Hatfield refers to this feeling of attachment as “companionate love,” defining it as “a feeling of happy togetherness with someone whose life has become deeply entwined with yours”(Hatfield 1988:191).
Just as men and women distinguish between feelings of romantic love and the sex drive, people distinguish between feelings of romance and those of attachment for a long term partner. Nisa, a !Kung Bushman woman of the Kalahari Desert, Botswana, explained the feeling of man/woman attachment, saying, “When two people are first together, their hearts are on fire and their passion is very great. After a while, the fire cools and that’s how it stays. They continue to love each other, but it’s in a different way—-warm and dependable”(Shostak 1981:268). The Taita of Kenya report that love comes in two forms, an irresistible longing, a “kind of sickness,” and a deep enduring affection for another (Bell 1995:158). Brazilians have a poetic proverb that distinguishes between these feelings, “Love is born in a glance and matures in a smile”(Rebhun 1995:252). For Koreans, “sarang” is a word close to the Western concept of romantic love; while “chong” is more like feelings of long term attachment. Abigail Adams described these feelings, writing to John Adams in 1793, “Years subdue the ardor of passion, but in lieu thereof friendship and affection deep-rooted subsists, which defies the ravages of time, and whilst the vital flame exists”(McCullough 2001).
Bowlby (Bowlby 1969; 1973) and Ainsworth (Ainsworth et al 1978) proposed that, to promote survival of the young, primates have evolved an innate attachment system designed to motivate infants to seek comfort and safety from their primary caregiver, generally their mother. More recently researchers have emphasized that this attachment system remains active throughout life and serves as a foundation for attachment between spouses as they raise children (Hazan and Diamond 2000; Hazan and Shaver 1987).
This parental attachment system has been associated with the activity of the neuropeptides, oxytocin (OT) in the nucleus accumbens and arginine vasopressin (AVP) in the ventral pallidum (Carter, 1992; Winslow et al 1993; Wang, Ferris and DeVries 1994; Young, Wang, & Insel, 1998; Lim, Murphy, & Young, 2004; Lim and Young 2004), although the brain’s opioid system (Moles et al 2004) and other neural systems are most likely also involved (Kendrick 2000). When vasopressin was injected intracerebroventricularly into virgin, labortory-raised male prairie voles, they began to defend the space around them from other males, an aspect of pair formation among prairie voles. When each was introduced to a female, he became instantly possessive of her as well (Winslow et al 1993; Wang, Ferris and DeVries 1994). Moreover, arginine vasopressin antagonists infused into the ventral pallidum prevented partner preference formation among male prairie voles, suggesting that V1a receptor activation in this region is necessary for their pairbond formation (Lim and Young 2004:1).
This distinct distribution of vasopressin receptors in the ventral forebrain seen in monogamous male prairie voles is also seen in monogamous California mice and monogamous marmoset monkeys, whereas promiscuous white-footed mice and promiscuous rhesus monkeys do not express this distribution of V1a receptors in the ventral pallidum (Wang et al 1997; Bester-Meredith et al 1999; Young 1999; Young et al 1997), further suggesting that vasopressin activity in this region of the brain’s reward system is directly associated with pairbonding and attachment behaviors (Lim, Murphy and Young 2004).
Oxytocin (OT) also stimulates the bonding process between a mother and her offspring (Carter 1992; Pedersen et al 1994) and between mating partners (Lim, Murphy and Young 2004). When oxytocin is administered intracerebroventricuarly, ovariectomized female prairie voles preferred the partner that was present at the time of infusion and forms a pairbond with him (Williams et al 1994). When an oxytocin receptor (OTR) antagonist is infused directly into the nucleus accumbens of a female prairie vole, it blocks partner preference and pairbond formation (Lim, Murphy and Young 2004; Young et al 2001).
A specific gene has also been associated with attachment behaviors and pairbonding. When this gene was manipulated to increase V1a receptors in the ventral pallidum, male prairie voles with increased V1aR expression exhibited heightened levels of social affiliation, formed a preference for a specific female, and began to cohabit with her, even though they had not mated with her (Pitkow et al 2001). When Lim, Young and colleagues introduced this gene into a male meadow vole (a promiscuous species), vasopressin receptors upregulated and he began to fixate on a particular female and mate exclusively with her, even though other females were available (Lim et al 2004).
Oxytocin and vasopressin appear to be associated with both partner preference and attachment/pairbonding while dopamine (and perhaps other monoamines) are related only to partner preference. So Young maintains that when monogamous prairie voles and individuals of other monogamous species engage in sex, they trigger the activity of vasopressin and oxytocin in specific reward centers of the brain;then dopamine in these reward centers enable males and females to prefer their current mating partner, thereby initiating attachment and pairbonding (Lim, Murphy and Young 2004). Moreover males of promiscuous species, who lack one link in this chain (V1a receptors in the ventral pallidum), may feel attraction but do not associate this pleasurable feeling with a specific female and do not initiate an attachment to her.
Data from the Demographic Yearbooks of the United Nations on 97 societies suggests the prevalence of this attachment system in humans: approximately 93.1% of women and 91.8% of men marry by age forty-nine (Fisher 1992). Moreover, when Fisher and colleagues examined a subset of their fMRI subjects who were in longer relationships, specifically those who were in love between 8-17 months, theyfound activation in the ventral pallidum, the brain region where activity has been linked with pairbonding and attachment behaviors in several other monogamous species.
The above studies suggest that a specific brain system is associated with pairbonding in humans and other mammals and that the neural correlates associated with this attachment system are largely distinct from those of the sex drive and romantic love. We propose that this attachment system is also jeopardized by serotonin-enhancing antidepressants.
Attachment and the sex drive: interactions
Oxytocin and vasopressin have complex relationships with the neurochemistry of the sex drive and serotonin. Some animal studies indicate that testosterone can elevate the activity of vasopressin (Villalba et al 1999; Delville et al 1996; Wang and De Vries 1995) and oxytocin (Arsenijevic and Tribollet 1998; Johnson et al 1991), thereby increasing attachment behaviors, including mutual grooming, scent marking and defending a nesting site (Winslow and Insel 1991). Likewise, elevated activity of oxytocin and vasopressin can increase testosterone production (Sirotkin and Nitray 1992; Homeida and Khalafalla 1990); and low activity of testosterone can reduce vasopressin activity (Wang and De Vries 1993).
Given this positive correlation between the chemistry of attachment and the sex drive, serotonin-enhancing antidepressants that inhibit the sex drive can potentially inhibit feelings of attachment as well. Moreover, elevated oxytocin can suppress central serotonin activity in the hypothalamus, hippocampus, midbrain and brainstem (Muir and Pfister 1998); elevated serotonin can suppress the activity of vasopressin (Ferris and Deville 1994); and elevated vasopressin can suppress the activity of serotonin (Schwarzberg et al 1981). These data also suggest that serotonin-enhancing antidepressants can potentially jeopardize feelings of attachment for a long term partner.
But other studies conflict with these data. Elevated serotonin can stimulate oxytocin release (Van de Kar et al 1998), potentially stimulating feelings of attachment. Moreover the sex drive and the attachment system have been negatively correlated. Increasing activity of testosterone can decrease the activity of vasopressin and oxytocin and elevated activity of vasopressin can decreasethe activity of testosterone (Thomas, Kim and Amico 1996). This inverse relationship between lust and attachment is dose-dependent; it varies depending on the quantities, timing and interactions among several hormones (Delville and Ferris 1995). But elevated activity of testosterone can reduce attachment behaviors.
Evidence of this negative correlation is seen in humans and other species. Men with high baseline levels of testosterone marry less frequently, have more adulterous affairs, commit more spousal abuse and divorce more often. As a man’s marriage becomes less stable, activity of testosterone rises. With divorce, male testosterone levels rise even more. Last, single men tend to have higher levels of testosterone than married men (Booth and Dabbs 1993). This negative relationship between testosterone and attachment behaviors has also been recorded in avian species. Male cardinals and blue jays flit from one female to the next; they do not remain to parent their young. These males have high levels of testosterone. Males of avian species that form monogamous pairbonds and remain with a mate to parent infants have much lower levels of testosterone during the parenting phase of the breeding season (De Ridder et al 2000; Raouf et al 1997). But when scientists surgically pump testosterone into monogamous male sparrows, these males abandon their nests, their young and their mates to court other females (Wingfield 1994).
This negative correlation between testosterone and attachment behaviors suggest that under some circumstances serotonin-enhancing antidepressants that suppress the sex drive can strengthen feelings of attachment in a long term relationship.
Attachment and romantic love: interactions
The biological relationships between the neural mechanisms for attachment and romantic love are equally varied and complex. Central dopamine and norepinephrine can stimulate the release of oxytocin and vasopressin (Galfi et al 2001; Ginsberg et al 1994), perhaps contributing to one’s growing feelings of attachment. But increasing activity of dopamine can also inhibit release of oxytocin (Seybold et al 1978; Vizi and Volbekas 1980); and increasing activity of oxytocin can interfere with dopamine and norepinephrine pathways (Kovacs et al 1990; Kovacs and Telegdy 1983; Schwarzberg et al 1981; Van de Kar et al 1998). Hence the chemistry of attachment may potentially jeopardize feelings of romance and the chemistry of romance can potentially inhibit feelings of attachment.
The biological relationships between the three brain systems for human mating and reproduction, the sex drive, romantic love and attachment, are dose-dependant and variable, depending on which brain regions are involved and on many other biological and environmental interacting factors. Nevertheless, serotonin-enhancing antidepressants can potentially produce a wide variety of effects on all three neural systems, including suppressing feelings of romantic love and altering feelings of attachment to a long term partner.
Orgasm as an attachment, romance and signaling device
Serotonin-enhancing antidepressants can potentially produce deleterious effects on other complex, largely unconscious (Grammar et al 2003), adaptive mechanisms for mate selection, pair formation and pair stability (Thomson and Fisher 2004).
Orgasm, for example, has many adaptive purposes. Among them, it facilitates feelings of attachment by elevating activity of oxytocin and vasopressin in both sexes (Carmichael et al 1987). So when individuals taking serotonin-enhancing antidepressants fail to achieve orgasm, they fail to stimulate in themselves the neural system associated with attachment and pairbonding. In this manner, these antidepressants can potentially endanger emotional bonding with a new partner and/or the stability of a long term partnership.
Sexual activity and orgasm may also make an individual more susceptible to falling in love. Genital stimulation and arousal produce elevated activity of dopamine and norepinephrine (Meston and Frohlic 2000; Pfaff 2005); orgasm also briefly increases norepinephrine levels in the blood (Meston and Frohlic 2000). So when individuals taking serotonin-enhancing antidepressants fail to initiate sexual activity, fail to become sexually aroused and fail to achieve orgasm, they fail to activate in themselves and their partner these neurotransmitter systems associated with romantic love.
Orgasm also may function as a device by which women assess potential mates (Miller 2000). Women do not reach orgasm with every coupling and the “fickle” female orgasm is currently regarded as an adaptive mechanism by which women distinguish between those partners who are willing to spend time and energy to give them pleasure and those who are abrupt, impatient and non-empathetic during intercourse. As the hypothesis is reasoned, those males who are willing to expend time and energy to please a woman sexually are also more likely to be committed, long term providers (Buss 2003). So when women take serotonin-enhancing antidepressants that inhibit their orgasmic response, they jeopardize their ability to assess the commitment level of a potential long term provider.
Women also use orgasm to assess an existing partnership. They report greater frequency of orgasm in long-term, committed relationships (Laumann, Paik and Rosen 1999) and the onset of anorgasmia in the middle of a long term mateship may potentially jeopardize the stability of this relationship.
Case Study: In one case, a thirty-two year old woman with recurrent depression and bulimia required relatively high doses of an SSRI to eliminate her chronic binging and purging. The medication led to loss of libido, delayed arousal and absent orgasm. But her long-term relationship also dissolved, due to the frustrations and conflicts engendered by the sexual side-effects of the SSRI medication.
Orgasm serves other purposes. Single women tend to have more orgasms with socially dominant, symmetrical males (Thornhill, Gangestad and Comer 1995). Social rank and facial and body symmetry are regarded as markers of fitness and good genes (Gangestad and Thornhill 1997), so single women who inhibit their ability to reach orgasm with these biologically fit men can jeopardize their social and genetic future.
Knocking out orgasm with serotonin-enhancing antidepressants can also jeopardize reproductive opportunities among married women engaging in clandestine affairs. Married women report frequent orgasms during their affairs (Baker and Bellis 1995). In these cases, orgasm may serve as a biological incentive to continue the extra-marital relationship, thereby increasing her likelihood of reaping extra resources and benefits for her and her children and/or increasing the likelihood of conceiving another child with better genes or different genes.
It has been theorized that orgasm evolved to serve female reproduction in three other ways (Buss 2003). The “paternity confidence hypothesis” proposes that female orgasm evolved to enable ancestral women to signal a partner that she was satisfied with him, thereby motivating him to remain with her to help support their forthcoming young. The “paternity confusion hypothesis” proposes that female orgasm evolved to motivate ancestral females to copulate with multiple partners, thereby confusing the identity of the true biological father of a forthcoming child and obliging each male to contribute to the survival of the infant (Hrdy 1999). The “sperm retention hypothesis” proposes that female orgasm evolved to transport sperm through the cervix, enhancing the probability of conception (Fox, Wolfs and Baker 1970).
The above data and theories suggest that female orgasm is a multi-purpose mechanism, designed to promote pairbonding with appropriate males, promote “extra pair copulations” to increase female fecundity, and enable a single woman to identify and win the best possible partner when she seeks a new relationship. All of these functions of female orgasm are jeopardized by serotonin-enhancing antidepressants.
Chemical clitoridectomy
Women who take serotonin-enhancing antidepressants also disrupt related evolutionary mechanisms for mate selection, pair formation and pair maintenance. The ring of nerves around the vaginal opening measures penis width and, by distending surrounding muscles, elevates sexual excitement. The clitoris also responds to minor variations in touch and angle, thereby measuring a partner’s skill, patience, determination and sensitivity to her needs (Miller 2000). By creating a chemical clitoridectomy, serotonin-enhancing antidepressants dull the responses of these devises (Frolich and Meston 2000), contribute to anorgasmia and diminish a woman’s ability to discern appropriate mating and marital partners. Anorgasmia may also motivate a woman to look beyond her primary relationship, even though this male may have superior genes, resources and parenting capabilities (Small 1995).
Serotonin-enhancing antidepressants may affect other subtle female mechanisms for courtship, mating and reproduction. At midcycle, ovulating women tend to have more erotic fantasies, initiate more sexual activity and experience a lower threshold for orgasm. They have a better sense of smell (Doty 1986) and are better able to discriminate healthy from unhealthy available males. At midcycle, women are also more likely to prefer men with higher bodily and facial symmetry and men who are creative, humorous and display other signs of good genes (Grammar et al 2003; Thornhill, Gangestad and Comer 1995; Miller 2000). Attraction to individuals with MHC histocompatibility and/or other immunological profiles may be linked to sex drive and/or sexual arousal too. These and many other courtship mechanisms evolved to aid mate assessment, mate choice and pair formation, and any or all of these brain responses could potentially be altered by serotonin-enhancing antidepressants.
Like drugs that blur vision, serotonin-enhancing medications may impair myriad female adaptive mechanisms, obscuring a woman’s ability to make appropriate mating choices, fall in love and/or sustain appropriate long-term reproductive relationships.
Penile Erection, Seminal Fluid and antidepressants
Men who take serotonin-enhancing antidepressants also inhibit an array of adaptive mechanisms that evolved to promote mate selection and partnership formation. For example, the penis may function as an internal courtship device (Miller 2000). With its width, length and turgidity, it stimulates the vagina to give pleasure; it also advertises psychological and physical fitness (Miller 2000). When men take antidepressants that produce impotence, they cripple these courtship functions.
The penis also deposits seminal fluid which contains dopamine and norepinephrine, as well as tyrosine, a building block of these catecholamines (Burch and Gallup, in press). These compounds do not pass through the blood-brain barrier. Nevertheless, when a man taking a serotonin-enhancing antidepressant fails to ejaculate, he fails to deposit these catecholamines in the vaginal tract, neurotransmitters that could potentially contribute to his partner’s feelings of romantic attraction to him.
Seminal fluid also contains several other mood-altering hormones, including testosterone, estrogen, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), chemicals that can also affect sexual desire and function (Clayton 2003). Gallup and colleagues have demonstrated that these and other chemicals in seminal fluid have antidepressant effects on women (Gallup et al 2002). So when a man fails to ejaculate, he potentially suppresses his ability to stimulate in his partner a positive mood that could potentially change her threshold for romantic attraction and/or deep attachment to him.
SSRI’s and psychological barriers to romance and marriage
Serotonin-induced sexual dysfunction can adversely affect feelings of romantic love and partner attachment in psychological ways as well. For example, some men and women taking these medications shy away from a liaison that could become romantic because they are afraid of their own poor performance in bed.
Case Study: In one case,a twenty-six year old man had panic attacks that required high doses of a serotonin-enhancing antidepressant. He soon experienced diminished libido and impotence. A handsome, personable, intelligent man, he was readily sought after by women. However, he ended several relation-ships because he was too embarrassed about his inability to perform sexually. Although he tried several other medications, he was only able to control his panic disorder with high doses of serotonin enhancers. He eventually retreated into a social life in which he avoided serious dating. When last evaluated, he still confined himself to non-sexual relationships with women.
Due to low libido, other patients on serotonin-enhancing antidepressants fail to become sexually attracted to a potential partner and incorrectly attribute their lack of sexual (and romantic) interest to personality deficits in this potential mate, thereby misappraising the viability of the relationship.
Still others fail to notice potential partners. Case Study: In one case, a patient in her late twenties had recurrent major depressions that were being controlled with an SSRI. She reported sexual side effects, including diminished sexual interest and absent orgasm. However, three to four weeks after the SSRI medication was reduced and an antidepressant with fewer sexual side effects was added, she noticed an increase in her sexual interest. When asked if she had noticed any change in her feelings of attraction to men, she said “I notice someone who is attractive now which I hadn’t before.”
SSRIs and fertility
These medications can also influence one’s genetic future. Case Study: In one case, a 35-year-old married woman with recurrent depression and generalized anxiety disorder was placed on an SSRI. She was not told about the potential negative sexual side effects of this medication. The drug relieved her depression and anxiety. However, she soon developed diminished libido and absent orgasm. This led her to conclude that she no longer loved her husband. She decided to divorce him but she kept her feelings to herself for several years, planning for the appropriate time to make this major life change. She eventually switched to an antidepressant with a low frequency of sexual side effects. On this new medication, her sexual desire and orgasmic function returned. She decided not to divorce her spouse. Soon after this, she conceived. Now she and her husband have a child. A serotonin-enhancing medication had affected not only her social life but her fertility.
These medications can also influence one’s genetic future in specific biological ways. Serotonin increases prolactin levels by inhibiting dopamine activity and stimulating prolactin releasing factors. Prolactin can impair fertility through several mechanisms, including suppressing hypothalamic GnRH release, suppressing pituitary FSH and LH release, and/or suppressing ovarian hormone production (Hendrick et al 2000). Also, Clomipramine, a strong serotonin-enhancing antidepressant, adversely affects sperm volume and motility (Maier and Koinig 1994).
The number and range of unconscious psychobiological mechanisms that have evolved to enable men and women to signal mating fitness, assess appropriate mating partners, pursue specific preferred individuals, and form and sustain a pairbond are largely unknown. But it is likely that many of these neural mechanisms are altered by serotonin-enhancing medications.
Conclusion
Homo sapiens has inherited three distinct, yet interrelated brain systems for courtship, mating, reproduction and parenting: the sex drive, romantic love and partner attachment. These neural systems can become active in any sequence. An individual may begin a casual sexual liaison with someone for whom they feel only sexual desire; then one evening they fall in love with him or her; then gradually they begin to feel deep attachment for this partner. Some couples begin their relationship with feelings of attachment instead; the man and woman become friends and achieve emotional union in the college dorm, at the office or in their social circle. With time, this attachment metamorphoses into romantic passion which then triggers lust. Still others fall in love with someone they hardly know; then they experience lust; last, they experience feelings of attachment. These three neural systems can also operate independently. An individual can feel deep attachment for a long-term spouse while they feel romantic passion for someone else while they feel the sex drive for an array of other individuals.
The flexible nature of these three brain mechanisms for reproduction and their complex, dynamic interactions suggest that anymedication that changes their chemical checks and balances is likely to alter an individual’s courting, mating and parenting tactics, ultimately affecting their fertility and genetic future.
Serotonin is the oldest known monoamine neurotransmitter; it has numerous receptors and many subtle functions. For example, activation of serotonin type 1a (5HT1a) receptors enhances sexual desire and lowers the threshold for ejaculation; activation of serotonin type 1b (5HT1b) and 1c (5HT1c) receptors decrease sexual desire and inhibit orgasm; and activation of serotonin type 2 (5HT2) and type 3 (5HT3) receptors impair all stages of sexual response in both men and women (Meston and Frolich 2000). Some 90% of these serotonin receptors are located in the body, where serotonin affects the smooth muscle of the vascular system, including the smooth muscle of the genitals.
Individuals vary in the sensitivity of these serotonin receptors (Saks 2000), as well as in many other aspects of serotonin production, synthesis and interaction with other bodily systems. Childhood experiences and current circumstances also affect one’s expression of this monoamine neurotransmitter. So individuals taking serotonin-enhancing antidepressants vary in their response to these medications, including their sexual side effects. In fact, data indicate that under the right circumstances serotonin-enhancing antidepressants can considerably improve several mental and physical disorders, including disorders that affect one’s romantic and marital relationships. Nevertheless, the Food and Drug Administration has warned Americans that these medications can have potentially harmful side effects, including severe restlessness, anxiety, hostility, insomnia and/or suicidal thinking, as well as emotional blunting and sexual dysfunction.
We believe that because there is a positive relationship between dopamine (associated with romantic love) and testosterone (linked to sexual desire and arousal) and because there is a negative relationship between serotonin and these catecholamines and the androgens, serotonin-enhancing antidepressants can also inhibit feelings of romantic love. Moreover, because serotonin-enhancing antidepressants have a negative impact on penile erection, sexual arousal, orgasm and other evolved psycho-biological courtship mechanisms, we believe these drugs can also negatively affect one’s ability to signal genetic and psychological fitness, assess and select potential mating partners, pursue preferred individuals and maintain stable pairbonds.
Harvard Medical School psychiatrist Joseph Glenmullen estimates that 75% of all patients on antidepressants, largely SSRIs, are “needlessly on these drugs”(Morais 2004:120). We propose that physicians who prescribe serotonin-enhancing antidepressants and individuals who plan to use these drugsshould bear in mind the broad, largely-unconscious and possibly deleterious effects of these medications.
BIBLIOGRAPHY
Abbott, A. (2002) Addicted. Nature 419(6910):872-874.
Ainsworth, MDS, MC Blehar, E Waters and S Wall (1978) Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Erlbaum.
Andersson, M. Sexual Selection. Princeton, NJ: Princeton University Press (1994).
Appararundaram, S., J. Huller, S. Lakhlani, and L. Jennes, (2002) Ovariectomy-induced alterations of choline and dopamine transporter activity in the rat brain. Society For Neuroscience Abstract Viewer/Itinerary Planner: p.368.20.
Arnow BA, JE Desmond, LL Banner, GH Glover, A Solomon, ML Polan, TF Lue, SW Atlas (2002) Brain activation and sexual arousal in healthy, heterosexual males. Brain 125(pt 5):1014-23.
Aron, A and E Aron (1991) “Love and sexuality.” In K McKinney and S Sprecher, Eds. Sexuality in close relationships. Hillsdale, NJ: Lawrence Erlbaum Associates.
Aron, A., Paris, M., & Aron, E. N. (1995) Falling in love: Prospective studies of self-concept change. Journal of Personality and Social Psychology, 69:1102-1112.
Aron, A, HE Fisher, DJ Mashek, G Strong, HF Li, and LL Brown (2005) Reward, Motivation and Emotion Systems Associated with Early-Stage Intense Romantic Love:an fMRI study. Journal of Neurophysiology 94:327-337
Arsenijevic Y and E. Tribollet (1998) Region-specific effect of testosterone on oxytocin receptor binding in the brain of the aged rat. Brain Research 785#1:167-70.
Ascher, JA, JO Cole, JN Colin, JP Feighner, RM Ferris, HC Fibiger, RN Golden, P Martin, WZ Potter, E Richelson and F Sulser (1995) Bupropion: A review of its mechanism of antidepressant activity. J. Clin Psychiatry 56#9:396-402.
Auger, A.P., J.M. Meredith, G.L. Snyder, and J.D. Blaustein, (2001) Oestradiol increases phosphorylation of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in female rat brain. J Neuroendocrinol 13(9): p. 761-8.
Baker, RR and M Bellis (1995) Human sperm competition. London: Chapman Hall.
Bartels, A, and S Zeki (2000). The neural basis of romantic love. NeuroReport, 11:3829-3834.
Bartels, A, and S Zeki (2004) The neural correlates of maternal and romantic love. NeuroImage, 21:1155-1166.
Beauregard M, J Levesque and P Bourgouin (2001) Neural correlates of conscious self-regulation of emotion. J Neurosci21(18):RC165
Becker, J.B., C. N. Rudick, et al.(2001) The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci 21(9): p. 3236-41.
Bell, J (1995) Notions of Love and Romance Among the Taita of Kenya. In W Jankowiak, Ed. Romantic Passion: A Universal Experience? New York: Columbia University Press.
Berridge, C.W., T.L. Stratford, S.L. Foote, and A.E. Kelley (1997) Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27(3):230-41.
Bester-Meredith JK, LJ Young and CA Marler (1999) Species
differences in paternal behavior and aggression in Peromyscus
and their associations with vasopressin immunoreactivity and
receptors. Horm Behav. 36:25-38.
Booth, A and JM Dabbs (1993) Testosterone and Men’s Marriages. Social Forces 72(2):463-477.
Bowlby, J (1969) Attachment and Loss: Attachment (Vol 1). New York: Basic Books
Bowlby, J (1973) Attachment and Loss: Separation (Vol 2). New York: Basic Books.
Breiter, HC, I Aharon, D. Kahneman, A Dale, P Shizgal (2001) functional Imaging of Neural Responses to Expectancy and Experience of Monetary Gains and Losses. Neuron 30:619-639.
Breiter, HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, and Hyman SE (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591-611.
Buffum, J, C Moser and D Smith (1988) “Street drugs and sexual function” In Handbook of Sexology. Vol 6: ThePharmacology and Endocrinology of Sexual Function. JMA
Sitsen, Ed. Elsevier Science Publishers B.V.
Buss, DM (2003) The Evolution of Desire: Strategies of Human Mating. Revised Edition. New York. Basic Books.
Burch, R L, & Gallup, G G, Jr. (in press). The psychobiology of human semen. In S. Platek & T. Shackelford (Eds.), Female Infidelity and Paternal Uncertainty. Cambridge University Press.
Cardinali DP, CA Nagle, E. Gomez and JM Rosner (1975) Norepinephrine turnover in the rat pineal gland: acceleration by estradiol and testosterone. Life Sci 16:11:1717-24.
Carmichael, MS, R Humbert, J Dixen, G Palmisano, W Greenleaf and JM Davidson (1987) Plasma oxytocin increases in the human sexual response. Journal of Clinical Endocrinology and Metabolism 64(1):27-31.
Carter, CS (1992) Oxytocin and Sexual Behavior. Neuroscience and Biobehavioral Reviews. 1(16):131-144.
Carter. CS, A DeVries, SE Taymans, RL Roberts, JR Williams and LL Getz (1997) “Peptides Steriods, and Pair Bonding” IN CS Carter, II Lederhendler and B Kirkpatrick, Eds. The Integrative Neurobiology of Affiliation. Annals of the New York Academy of Sciences. 807:260-272. New York: The New York Academy of Sciences.
Clayton, Ah, E.D. McGarvey, J. Warnock et al (2000) Bupropion as an antidote to SSRI-iduced sexual dysfunction. Poster presented at the New Clinical Drug Evaluation Unit Program (NCDEU), Boca Raton, FL.
Clayton, AH (2003) Sexual function and dysfunction in Women. Psychiatric Clinics of North America 26:673-682.
Clayton, AH, JF Pradko, HA Croft, B Montano et al (2002) Prevalence of sexual dysfunction among newer antidepressants. J. Clin Psychiatry 63:357-366.
Coleman, CC, LA. Cunningham, VJ. Foster, SR Batey, RMJ Donahue, TL Houser and JA Ascher (1999) Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of buproprion sustained release and sertraline treatment. Annals of Clinical Psychiatry 11#$:205-215.
Colle, L M and R A Wise (1988) Facilitory and Inhibitory Effects of Nucleus Accumbens Amphetamine on Feeding. In The Mesocorticolimbic Dopamine System, P.W. Kalivas and C.B. Nemeroff, eds. Annal of the New York Academy of Science, Vol 537.
Coull, J, (1998) Neural correlates of attention and arousal Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 55: p. 343-361.
Curtis, JT and Z Wang (2003) Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster) Physiology and Behavior 80:95-101.
Creutz, L.M. and M.F. Kritzer (2002) Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol 446(3): p. 288-300.
David V, L Segu, MC Buhot, M Ichaye, and P Cazala (2004) Rewarding effects elicited by cocaine microinjections into the ventral tegmental area of C57BL/6 mice: involvement of dopamine D(1) and serotonin(1B) receptors. Psychopharmacology 174(3):367-75.
Davidson, R J (1994) Complexities in the Search for Emotion-Specific Physiology. The Nature of Emotion: Fundamental Questions, P. Ekman P. and R.J. Davidson, eds. New York: Oxford University Press.p 237-242.
Delgado MR, LE Nystrom, C Fissel, DC Noll, and JA Fiez (2000) Tracking the Hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol 84:3072-3077.
Delville Y and C.F. Ferris (1995) Sexual differences in vasopressin receptor binding within the ventrolateral hypothalamus in golden hamsters. Brain Research Vol 68#1:91-96.
Delville Y, KM Mansour and CF Ferris (1996) Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav 60#1:25-29.
De Ridder, E Pinxten and M Eens (2000) Experimental evidence of a testosterone-induced shift from paternal to mating behaviour in a facultatively polygynous songbird. Behavioral Ecology and Sociobiology 49#1:24-30.
Dixson, AF (1998) Primate sexuality. Oxford University Press, Oxford.
Dluzen DE, VD Ramirez, CS Carter and LL Getz (1981) Male vole urine changes luteinizing hormone-releasing hormone and norepinephrine in female olfactory bulb. Science 212:573-575.
Done CJ and T Sharp (1992) Evidence that 5-HT2 receptor activation decreased noradrenaline release in rat hippocampus in vivo. Br. J. Pharmacol 107:240-5.
Doty, Richard L. (1986) “Gender and endocrine-related influences on human olfactory perception.” In Herbert L. Meiselman and Richard S. Ravlin, Clinical Measurement of Taste and Smell. New York: Macmillan.Pp377-413
Edwards, JN and A Booth, (1994) “Sexuality, Marriage, and Well-Being: The Middle Years” IN A.S. Rossi, (Ed.) Sexuality across the life course. Chicago: University of Chicago Press.
Elliott, R, JL Newman, OA Longe, JFW Deakin (2003) Differential Resonse patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J. Neuroscience 23(1):303-307.
Etgen AM, HP Chu, JM Fiber, GB Karkanias, and JM Morales (1999) Hormonal integration of neurochemical and sensory signals governing female reproductive behavior. Behav Brain Res 105(1):93-103.
Etgen, A. and JC Morales (2002) Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexually receptive female rats. J Neuroendocrinol 14(3):213-8.
Etgen, AM (2002) Estrogen Regulation of Neurotransmitter and Growth Factor Signaling in the Brain. In Hormones, Brain and Behavior,Pfaff DW, Arnold A., Etgen A, Fahrbach S, and Rubin R., Eds. 5 Volumes, Academic Press. p. 381-440.
Fabre-Nys C. (1998) Steroid control of monoamines in relation to sexual behaviour. Rev Reprod 3#1:31-41.
Fernandez BE, NA Vidal and AE Dominguez (1975) Action of the sexual hormones on the endogenous norepinephrine of the central nervous system. Rev Esp Fisiol 31#4;305-7.
Ferrari, F and D Giuliani (1995) “Sexual attraction and copulation in Male Rats: effects of the dopamine agonist SND 919.” Pharmacology Biochemistry and Behavior 50:1:29-34.
Ferris, CF and Y Delvile (1994) Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology 19:(5-70):593-601.
Fiorillo, CD, PN Tobler and W Schultz (2003) Discrete coding of reward probablility and uncertainty by dopamine neurons. Science299:1898-1901.
Fisher, HE (1992) Anatomy of Love: The Natural History of Monogamy, Adultery and Divorce. New York: WW Norton
Fisher, HE. (1998) Lust, Attraction, and Attachment in Mammalian Reproduction. Human Nature Vol 9#1:23-52.
Fisher, HE (1999) The First Sex: The National Talents of Women and How They are Changing the World. New York: Random House
Fisher, HE, A Aron, D Mashek, H Li, G Strong and LL Brown (2002a) The Neural Mechanisms of Mate Choice: A Hypothesis. Neuroendocrinology Letters Suppl 4, vol 23:92-97.
Fisher, HE, A Aron, D Mashek, G Strong, H Li and LL Brown. (2002) Defining the Brain Systems of Lust, Romantic Attraction and Attachment. Archives of Sexual Behavior, 31# 5: 413-9.
Fisher, HE., A Aron, D Mashek, G Strong, H Li and LL Brown (2003) Early stage intense romantic love activates cortical-basal-ganglia reward/motivation, emotion and attention systems: an fMRI study of a dynamic network that varies with relationship length, passion intensity and gender. Poster presented at the Annual Meeting of the Society For Neuroscience, New Orleans, November 11.
Fisher, HE (2004). Why we love: The nature and chemistry of romantic love. New York: Henry Holt.
Fisher, HE and JA Thomson, Jr.(2004) “Do the Sexual Side Effects of most Antidepressants Jeopardize Romantic love and Marriage?” Paper presented in symposium: Sex, Sexuality, and Serotonin. Annual meeting of the American Psychiatric Association (May 1)
Fisher, H, A Aron, D Mashek, G Strong, H Li and LL Brown. (2005) Motivation and emotion systems associaed with romantic love following rejection: An fMRI study. Poster presented at the annual meeting of the Society for Neuroscience, Washington, DC, Nov 15.
Flament, MF, JL Rapoport, and CL Berg (1985) Clomipramine Treatment of Childhood Obsessive-Compulsive Disorder: a Double-Blind Controlled Study. Arch. Gen. Psychiatry 42:977-986.
Fox, CA, HS Wolfs and JA Baker (1970) Measurement of Intra-Vagina and Intra-Uterine Pressures During Human Coitus by Radio-Telementry. Journal of Reproduction and Fertility 22:243-51.
Fraley, GS (2002) Immunolesion of hindbrain catecholaminergic projections to the medial hypothalamus attenuates penile reflexive erections and alters hypothalamic peptide mRNA. J Neuroendocrinol 14(5): p. 345-8.
Frankel, J (2004) Reviving Romance. The New York Times, F4, Letters, May 11.
Frohlich, PF and CM Meston (2000) Evidence that serotonin affects female sexual functioning via peripheral mechanisms. Physiol Behav.71:383-93.
Galfi, M, T. Janaky, R. Toth, G. Prohaszka, A. Juhasz, C. Varga and F.A. Laszlo (2001) Effects of dopamine and dopamine-active compounds on oxytocin and vasopressin production in rat neurohypophyseal tissue cultures. Regul Pept Vol 98#1-2:49-54.
Gallup, GG Jr, RL Burch and SM Platek ((2002) Does Semen Have Atidepressant properties? Archives of Sexual Behavior 13:26:289-293.
Gangestad, S.W. and R. Thornhill (1997) The evolutionary psychology of extrapair sex: the role of fluctuating asymmetry. Evolution and Human Behavior 18#2:69-88.
Gibbs, RB (2000) Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience 101(4): p. 931-8.
Gingrich B, Liu Y, Cascio C, Z, and Insel TR. (2000) Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 114: 173-183.
Gold, JI (2003) Linking reward expectations of behavior in the basal ganglia. Trends in Neuroscience 26(1):12-14.
Gonzaga GC, Keltner D, Londahl EA, and Smith MD. Love and the commitment problem in romantic relations and friendship. J Pers Soc Psychol 81: 247-262, 2001.
Gingrich B, Liu Y, Cascio C, Wang Z, and Insel TR. (2000) Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 114: 173-183, 2000.
Ginsberg, SD, PR Hof, WG Young and JH Morrison (1994) Noradrenergic innervation of vasopressin-and oxytocin-containing neurons in the hypothalamic paraventricular nucleus of the macaque monkey: quantitative analysis using double-label immunohistochemistry and confocal laser microscopy. J. Comp Neurol 341#4:476-91.
Gonzalez MI, F. Farabollini, E. Albonetti, and CA Wilson (1994) Interactions between 5-hydroxytryptamine (5-HT) and testosterone in the control of sexual and nonsexual behaviour in male and female rats. Pharmacol Biochem Behav 47#3:591-601.
Grammar, K, B. Fink, AP Moller, R. Thornhill (2000) Darwinian aesthetics: sexual selection and the biology of beauty. Biol. Rev. 78:385-407.
Griffin, MG and GT Taylor (1995) Norepinephrine Modulation of Social Memory: Evidence for a Time-Dependent Functional Recovery of Behavior. Behavioral Neuroscience 109#3:466-473.
Harris, H. (1995) “Rethinking Heterosexual Relationships in Polynesia: A Case Study of Mangaia, Cook Island” in W. Jankowiak, Ed. Romantic Passion: A Universal Experience? New York: Columbia University Press.
Harvey, PH and AH Harcourt (1984) Sperm competition, testes size, and breeding systems in primates. In R. Smith (Ed), Sperm competition and the evolution of animal mating systems, pp 589-659. New York: Academic Press.
Hatfield, E and RL Rapson (1996) Love and Sex: cross-cultural perspectives. Needham Heights, MA: Allyn and Bacon
Hatfield, E and S Sprecher (1986) Measuring passionate love in intimate relationships. Journal of Adolescence 9:383-410.
Hatfield, E (1988) Passionate and Companionate Love. In R.J. Sternberg and M.sL. Barnes, Eds. The Psychology of Love. New Haven, CT: Yale University Press.
Hazan, C and LM Diamond (2000) The place of attachment in human mating. Review of General Psychology 4:186-204.
Hazan, C and PR Shaver (1987) Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology52:511-524.
Heaton, J P (2000) Central neuropharmacological agents and mechanisms in erectile dysfunction: the role of dopamine. Neuroscience & Biobehavioral Reviews. 24,#5:561-569.
Hendrick V, Gitlin M, Altshuler L, Korenman S.(2000) Antidepressant medications, mood and male fertility. Psychoneuroendocrinology25#1:37-51(15).
Herbert, J (1996) Sexuality, stress and the chemical architecture of the brain. Ann Review of Sex Research 7:1-44.
Hollander, E, M Fay, B Cohen, R Campeas, JM Gorman and M R Liebowitz (1988) Serotonergic and Noradrenergic Sensitivity in Obsessive-Compulsive Disorder: Behavioral Findings. American Journal of Psychiatry 145#8:1015-1017.
Hrdy, SB (1999) Mother nature: A history of mothers, infants and natural selection. New York: Pantheon.
Homeida AM and AE Khalafalla (1990) Effects of oxytocin and an oxytocin antagonist on testosterone secretion during the oestrous cycle of the goat (Capra hircus). J. Reprod Fertil 89#1:347-50.
Hull, E.M; Du, J; Lorrain, D.S; and L. Matuszewich (1995) “Extracelular dopamine in the medial preoptic area: implicatons for sexual motivation and hormonal control of copulation.” Journal of Neuroscience Vol 15: Issue 11:7465-7471.
Hull, E., R. Meisel, and B.D. SAChs, (2002) Male Sexual Behavior. In Hormones, brain, and behavior, D.W. Pfaff, et al., Eds, Academic Press: Boston. 1-139.
Hull, E., J. Du, D. Lorrain, and L. Matuszewich, (1997) Testosterone, preoptic dopamine, and copulation in male rats. Brain Res Bull.44:327-333.
Hull, EM, DS Lorrain, J Du, L Matuszewick, LA Lumley, SK Putnam, and J Moses (1999) Hormone-neurotransmitter interactions in the control of sexual behavior. Behavioural Brain Research 105#1:105-16.
Insel, TR, J Zohar, C Benkelfat and DL Murphy (1990) Serotonin in obsessions, compulsions, and the control of aggressive impulses. Annuals of the New York Academy of Sciences 600:574-586.
Insel, TR, EA Mueller, I Alterman, M Linnoila and DL Murphy (1985) Obsessive-compulsive disorder and serotonin: is there a connection? Biological Psychiatry 20:1174-1188.
Jankowiak, WR and EF Fischer (1992) A Cross-Cultural Perspective on Romantic love. Ethnology 31(2): 149.
Jankoiwiak, W (1995) “Introduction” in W. Jankowiak, Ed. Romantic Passion: A Universal Experience? New York: Columbia University Press.p 1-19.
Johnson AE, H Coirine, TR Insel and BS McEwen (1991) The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by testosterone and its metabolites. Endocrinology 128#2:891-6.
Jones, TJ, G Dunphy, A Milsted and D Ely (1998) Testosterone effects on renal norepinephrine content and release in rats with different Y chromosomes. Hypertension 32#5:880-885.
Kalivas PW and Duffy P.(1998) Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem70: 1497-1502.
Karama S, AR Lecours, JM Leroux, P Bourgouin, G Beaudoin, S Joubert and M Beauregard (2002) Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 16(1):1-13
Kawashima S and K Takagi (1994) Role of sex steroids on the survival, neuritic outgrowth of neurons, and dopamine neurons in cultured preoptic area and hypothalamus. Horm Behav 28#4:305-12.
Kendrick, KM, EB Keverne, BA Baldwin and DF Sharman (1986) Cerebrospinal fluid levels of acetylcholinestrase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep.
Neuroendocrinology 44:149-156.
Kendrick, KM (2000) Oxytocin, motherhood and bonding Experimental Physiology 85S:111S-124S.
Kiyatkin, EA (1995) Functional Significance of Mesolimbic Dopamine. Neuroscience & Biobehavioral Reviews. 19(4):573-98.
Kovacs GL, Z Sarnyai, E. Barbarczi, G Szabo and G Telegdy (1990) The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity.Neuropharmacology 29#4:365-8.
Kovacs G, and G Telegdy (1983) Effects of oxytocin, des-glycinamide-oxytocin and anti-oxytocin serum on the alpha-MPT-induced disappearance of catecholamines in the rat brain. Brain Res268#2:307-14.
Lauman, EO, A Paik and RC Rosen (1999) Sexual dysfunction in the United States: prevalence and predictors. JAMA 281(6): 537-44.
Lauwereyns J, Takikawa Y, Kawagoe R, Kobayashi S, Koizumi M, Coe B, Sakagami M, and Hikosaka O (2002) Feature-based anticipation of cues that predict reward in monkey caudate nucleus. Neuron 33:463-473.
Lim, MM, AZ Murphy and LJ Young (2004) Ventral striatopallidal Oxytocin and Vasopressin V1a receptors in the Monogamous Prairie Vole (Microtus ochrogaster). The Journal of Comparative Neurology 468:555-570
Lim, MM and LJ Young (2004) Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125:35-45..
Lim, MM, ZX Wang, DE Olazabal, XH Ren, EF Terwilliger and LJ Young (2004) Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature, 429(6993):754-757
Liu, Yian-Cheng, B.D. Sachs and J.D. Salamone (1998) Sexual behavior in male rats after radiofrequency or dopamine-depleting lesions in nucleus accumbens. Pharmacology Biochemistry and Behavior 60#1:585-592.
Liu Y and Wang ZX. (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121: 537-544, 2003.
Maier U and F Koinig ( 1994) Andrological findings in young patients under long-term antidepressive therapy with clomipramine. Psychopharmacology 116:357-359.
Marazziti, D, HS Akiskal, A Rossi and GB Cassano (1999) Alteration of the platelet serotonin transporter in romantic love. Psychological Medicine 29:741-745.
Marrocco, R, and M Davidson (1996) Neurochemistry of Attention, In Mechanisms of Attention, R. Parasuraman, Ed. p.35-50.
Martin-Soelch, C, Leenders K.L., Chevalley, A.F., Missimer, J., Kunig, G., Magyar, S., Mino, A., Schultz, W. (2001) Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Research Reviews 36:139-149.
Mayerhofer A, RW Steger, G Gow and A Bartke (1992) Catecholamines stimulate testicular testosterone release of the immature golden hamster via interaction with alpha-and beta-adrenergic receptors. Acta Endocrinol 127#6:526-30.
McBride WJ, Murphy JM, and Ikemoto S. (1999) Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101: 129-152.
McCullough. D (2001) John Adams. New York: Simon and Schuster
Meikle, A, J Stringham, D Bishop and D West (1988) “Quantitating genetic and nongenetic factors influencing androgen production and clearance rates in men.” Journal of clinical Endocrinology Metabolism 67:104-9.
Meloy, JR and HE Fisher (2005) Some Thoughs on the Neurobiology of Stalking. Journal of Forensic Sciences 50#6:1472-1480.
Meston, CM and PF Frohlic (2000) “The Neurobiology of Sexual Function.” Arch. Gen. Psychiatry 57:1012-1030.
Miller, GF (2000) The Mating Mind: How sexual choice shaped the evolution of human nature. New York: Doubleday
Moles, A, BL Kieffer and FR D’Amato (2004) Deficit in attachment behavior in mice lacking the u-opioid receptor gene. Science304:1983-1985.
Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, Kilpatrick MR, and Wightman RM (2004) Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci 24: 1754-1759.
Montejo, AL, G Llorca, JA Izquierdo and F Rico-Vallademoros (2001) Incidence of Sexual Dysfunction Associated with Antidepressant Agents: A Prospective Multicenter Study of 1022 Outpatients. J. Clin Psychiatry 62(3):1020.
Morais, RC (2004) “Prozac Nation: Is the party over?” Forbes Sept 6, p 119-124.
Muir JL and HP Pfister (1998) Psychological stress and oxytocin treatment during pregnancy affect central norepinephrine, dopamine and serotonin in lactating rats. Int. J. Neurosci 48(3-4):191-203.
Netter, P, J. Hennig, B. Meier and S. Rohrmann (1998) Testosterone as an indicator of altered 5-HT responsivity in aggressive subjects. European Psychiatry 13#4:181s.
Frankel, J (2004) Reviving Romance. New York Times, May 11, F4
Nyborg, H (1994) Hormones, sex and society. Westport, Ct: Praeger.
O’Connor, A (2004) Has the romance gone? Was it the drug? The New York Times, Science Section, F8, May 4.
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, and Dolan RJ (2004) Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304:452-454.
Panksepp, J (1998) Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press.
Pedersen, CA, JD Caldwell, C Walker, G Ayers and GA Mason (1994) Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral Neuroscience 108(6):1163-1171.
Pfaff, D.W., Drive: Neural and Molecular Mechanisms for Sexual Motivation. 1999, Cambridge, Mass.: The MIT Press.
Pfaff, D (2005) Brain arousal and information theory. Cambridge, MA: Harvard University Press.
Pitkow, LJ, CA Sharer, X Ren, TR Insel EF Terwilliger and LJ Young (2001) Facilitation of Affiliation and Pair-Bond Formation by Vasopresin Receptor Gene Transfer into the Ventral Forebrain of a Monogamous Vole. Journal of Neuroscience, 21:#18:7392:7396.
Posner, M. and S. Petersen, (1990) The attention system of the human brain. Annual Review of Neuroscience 13: 25-42.
Post, R. M., S. R. B. Weiss and A. Pert (1988) Cocaine-induced Behavioral Sensitization and Kindling: Implications for the Emergence of Psychopathology and Seizures. In The Mesocorticolimbic Dopamine System, P.W. Kalivas and C.B. Nemeroff, eds. Pp. 292-308. Annal of the New York Academy of Sciences, Vol 537. New York: The New York Academy of Sciences.
Raouf, SA, PG Parker, ED Ketterson, V Nolan Jr. and C Ziegenfus (1997) Testosterone affects reproductive success by influencing extra-pair fertilizations in male dark-eyed juncos (Aves: Junco hyemalis) Proceedings of the Royal Society of London – B. Biological Sciences. 264:#1388:1599-1603.
Rebhun, LA (1995) “Language of Love in Northeast Brazil” In Jankowiak, William, ed. Romantic Passion: A Universal Experience? New York: Columbia University Press.
Reno, PL, RS Meindl, MA McCollum and CO Lovejoy (2003) Sexual dimprphism in Australopithecus afarensis was similar to that of mondern humans. Proceedings of the National Academy of Sciences 10:1073.
Robbins, T, S Granon, J Muir, P Durantou, A Harrison, and B Everitt (1998) Neural systems underlying arousal and attention: Implications for drug abuse. Annals of the New York Academy of Sciences, 846:222-237.
Robbins, TW and BJ Everitt (1996) Neurobehavioural Mechanisms of Reward and Motivation. Current Opinion in Neurobiology 6#2:228-236.
Robinson DL, Heien ML, and Wightman RM (2002) Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci 22:10477-10486.
Rosen, RC, RM Lane and M Menza (1999) Effects of SSRIs on sexual function: A critical review. Journal of Clinical Psycopharmacology 19(1):67-85.
Saks, BR (2000) Sex receptors: mechanisms of drug action via biochemical receptors on sexual response in women. J. Sex Education and Therapy 20:33-35.
Salamone, J. D. (1996) The Behavioral Neurochemistry of Motivation: Methodological and Conceptual Issues in Studies of the Dynamic Activity of Nucleus Accumbens Dopamine. Journal of Neuroscience Methods. 64(2):137-49.
Schultz, W (2000) Multiple Reward Signals in the Brain. Nature Reviews. Neuroscience 1:199-207.
Schultz W, P Dayan, and P Read Montague (1997) A neural substrate of prediction and reward. Science 275:1593-1598.
Schwarzberg H, GL Kovacs, G Szabo and G Telegdy (1981) Intraventricular administation of vasopressin and oxytocin effects the steady-state levels of serotonin, dopamine and norepinephrine in rat brain. Endocrinol Exp 15#2:75-80.
Segraves RT, H Croft, R Kavoussi et al (2001) Bupropion sustained release (SR) for treatment of hypoactive sexual desire disorder (HSDD) in nondepressed women. J. Sex Marital Ther 27:303-16.
Seybold VS, JW Miller and PR Lewis (1978) Investigation of a dopaminergic mechanism for regulating oxytocin release. J Pharmacol Exp Ther 207(2):605-10.
Sherwin, Barbara B (1994) Sex hormones and psychological functioning in postmenopausal women. Experimental Gerontology29#3/4:423-430.
Semendeferi, K, H. Damasio, R. Frank and GW Van Hoesen (1997) The evolution of the frontal lobes: a volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. Journal of Human Evolution 32:375-388.
Shaver P, Schwartz J, Kirson D, and O’Connor C. Emotion knowledge: further exploration of a prototype approach. J Pers Soc Psychol 52: 1061-1086, 1987.
Shostak, M. (1981) Nisa: The life and words of a !Kung woman. Cambridge, MA: Harvard University press.
Sirotkin AV and J. Nitray (1992) The influence of oxytocin, vasopressin and their analogues on progesterone and testosterone production by porcine granulosa cells in vitro. Ann Endocrinol (Paris) 53#1:32-6.
Small, M (1995) What’s Love Got to Do With It? The Evolution of Human Mating. New York: Doubleday.
Stahl, SM (2000) Essential Psychopharmacology 2nd Ed. New York: Cambridge University Press.
Sundblad C and E Eriksson (1997) Reduced extracellular levels of serotonin in the amygdala of androgenized female rats. Eur Neuropsychopharmacol 7#4:253-9.
Szezypka MS, QY Zhou and RD Palmiter (1998) Dopamine-stimulated sexual behavior is testosterone dependent in mice. Behav Neurosci112#5:1229-35.
Tennov, D. (1979) Love and Limerence: The Experience of Being in Love. New York: Stein and Day.
Thomson, JA Jr and HE Fisher (2004) “Do the Sexual Side-Effects of Antidepressants Jeopardize Romantic Love and Marriage?” Paper presented at the annual meeting of the Human Behavior and Evolution Society, July 21, Berlin.
Thomas A, NB Kim and JA Amico (1996) Sequential exposure to estrogen and testosterone (T) and subsequent withdrawal of T increases the level of arginine vasopressin messenger ribonucleic acid in the hypothalamic paraventricular nucleus of the female rat. Journal of Neuroendocrinology vol 8#10:793-800.
Thoren, P., M. Asberg, and L. Bertilsson (1980) Clomipramine Treatment of Obsessive Disorder: Biochemical and Clinical Aspects. Arch. Gen. Psychiatry 37:1289-1294.
Thornhill, R, SW Gangestad and R Comer (1995) Human female orgasm and mate fluctuating asymmetry. Animal Behavior 50:1601-15.
Tiihonen, J., J.T. Kuikka, K.A. Bergstrom, J. Karhu, H. Viinamiki, J. Lehtonen, T. Hallikainen, J. Yang and P. Hakola (1997) Single-photon emission tomography imaging of monoamine transporters in impulsive violent behaviour. European Journal of Nuclear Medicine Vol 24#10:1253-1260.
Tiihonen J, J Kuikka, J Kupila, K Partanen, P Vainio. J. Airaksinen, M Eronen, T Hallikainen, J Paanila, I Kinnunen, J Huttunen (1994) Increase in cerebral blood flow of right prefrontal cortex in men during orgasm. Neuroscience Letters 170:241-243.
Van Bockstaele, E.J., A. Saunders, P. Telegan, and M.E. Page (1999) Localization of mu-opioid receptors to locus coeruleus-projecting neurons in the rostral medulla: morphological substrates and synaptic organization. Synapse, 1999. 34(2):154-67.
Van Bockstaele, E.J., J. Peoples, and P. Telegan (1999) Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 412(3):410-28.
Van Bockstaele, E.J., V.A. Pieribone, and G. Aston-Jones (1989) Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. J Comp Neurol. 290(4):561-84.
Van de Kar, LD, AD Levy, Q Li, and MS Brownfield (1998) A comparison of the oxytocin and vasopressin responses to the 5-HT1A agonist and potential anxiolytic drug alnespirone (S-20499). Pharmacol Biochem Behav 60#3:677-83.
Van Goozen, S, VM Wiegant, E Endert, FA Helmond and N. E. Van de Poll (1997) Psychoendocrinological assessment of the menstrual cycle: the relationship between hormones, sexuality, and mood. Archives of Sexual Behavior 26#4:359-382.
Van de Kar, LD, AD Levy, Q Li and MS Brownfield (1998) A comparison of the oxytocin and vasopressin responses to the 5-HT1A agonist and potential anxiolytic drug alnespirone (S-20499) Pharmacol Biochem Behav 60(3):677-83.
Villalba D, CJ Auger and GJ De Vries (1999) Antrostenedione effects on the vasopressin innervaton of the rat brain. Endocrinology140#7:3383-6.
Vizi ES and V Volbekas (1980) Inhibition by dopamine of oxytocin release from isolated posterior lobe of the hypophysis of the rat: disinhibitory effect of beta-endorphin/enkephalin. Neuroendocrinology 31(1):46-52.
Walker, PW, JO Cole, EA Gardner, et al (1993) Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. Jounal of Clinical Psychiatry 54:459-65.
Wang Z, and GJ De Vries (1993) Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster) Brain Res. 63(1):156-60.
Wang ZX, CF Ferris and GJ De Vries (1994) The role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proceedings of the National Academy of Sciences (USA) 91:400-404.
Wang, Z, and GJ De Vries (1995) Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. Journal of Neuroendocrinology 7#1:827-31.
Wang Z, D Toloczko, LJ Young, K Moody, JD Newman, and TR Insel (1997) Vasopressin in the forebrain of common marmosets (Calithrix jacchus): studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res 768:147-156.
Wang, Z, Yu, G, Cascio, C, Liu, Y, Gingrich, B, & Insel, TR (1999). Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behavioral Neuroscience, 113(3):602-11.
Wenkstern, D, JG Pfaus and HC Fibiger (1993) Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Research 618:41-46.
Wersinger SR and EF Rissman (2000) Dopamine activates masculine sexual behavior independent of the estrogen receptor alpha. J. Neuroscience 20#11:4248-54.
West, CHK, AN Clancy and RP Michael (1992) “Enhanced responses of nucleus accumbens neurons in male rats to novel odors associated with sexually receptive females.” Brain Research 585:49-55.
Williams, JR, TR Insel, CR Harbaugh and CS Carter (1994) Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus Ochrogaster) Journal of Neuroendocrinology 6(3):247-250.
Wingfield, J.C. (1994) “Hormone-behavior interactions and mating systems in male and female birds.” RV Short and E Balaban, Eds. The Differences Between the Sexes. Cambridge University Press. pp. 303-330.
Winslow JT, N Hastings, CS Carter, CR Harbaugh and TR Insel (1993) A role for central v asopressin in pair bonding in monogamous prairie voles. Nature 365:545-548.
Winslow JT and TR Insel (1991) Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration J. Neurosci 11#7:203-8.
Wise, R. A. 1988 Psychomotor Stimulant Properties of Addictive Drugs. In The Mesocorticolimbic Dopamine System, Kalivas, PW, and CB Nemeroff, eds. Pp. 228-234. Annal of the New York Academy of Science, Vol 537. New York: The New York Academy of Sciences.
Wise, RA (1989) Brain Dopamine and Reward. Annual Review of Psychology 40:191-225.
Wise, RA (1996) Neurobiology of Addiction. Curr. Opin. Neurobiol. 6#2:243-251.
Wise, RA and DC Hoffman DC (1992) Localization of drug reward mechanisms by intracranial injections. Synapse 10: 247-263.
Young, LJ, JT Winslow, R Nilsen and TR Insel (1997) Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci 111:599-605.
Young LJ, Z WAng and TR Insel (1998) Neuroendocrine bases of monogamy. TINS 21#2:71-75.
Young, LJ (1999) Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav 36:212-221.
Young, LJ, MM Lim, B Gingrich and TR Insel (2001) Cellular mechanisms of social attachment. Horm Behav 40:133-138.